Anesthesia Intraoral Module (AIM)
(Direct Pharyngeal Oxygenation)
AIM’s pulmonary oxygen delivery far exceeds face masks and rivals High-Flow Nasal Cannulas. Its efficiency, portability, rapid setup, MRI compatibility and the integrated bite-block make it a smart choice for the high-volume and complex NORA procedures.
FDA clears AIM for immediate use in NORA procedures.
Oxygen Powerhouse
AIM delivers oxygen closer to the tracheal inlet, bypassing the oro-nasal dead space ensuring high pulmonary oxygenation.
During testing by an FDA-approved, third-party lab, an oxygen sensor at tracheal level captured 58% FiO2 with a commonly used oxygen mask, and a significantly higher 96% oxygen delivery with AIM (100% FiO2 with higher-flows).
Higher oxygen delivery ensures a favorable outcome in patients with low oxygen reserves (obesity, CHF, COPD, low EF or overly sedated, hypoventilating patients).


AIM Vs. High Flow Nasal Cannula
AIM’s pulmonary oxygen delivery rivals the High Flow Nasal Cannulas, offering a financially feasible alternative for the high volume NORA practice.
A recent study from Baylor University demonstrated pharyngeal oxygen delivery at 10 l/min superior to the high-flow nasal cannula (HFNC) at 60 l/min in a manikin model.

Improved Capnography
AIM captures pharyngeal CO2 with prominent capnography waveforms. The smaller waveforms of a hypoventilating patient (from upper airway obstruction, over-sedation) are easier to identify. An early alert can prevent a hypoxic event in remote hospital locations.


Single-Step Oral Airway Placement
Oral airway placement for upper airway obstruction is a multi-step process (remove face mask, pry the jaw open of a sedated, often uncooperative patient, place oral airway, replace face mask).
Full oral cavity access and uninterrupted oxygenation with AIM enables oral airway placement in a single-step, minimizing procedural interruptions.
Seamless Conversion to General Anesthesia
Emergent conversion to general anesthesia may become necessary (excessive patient movement, extreme pain, or respiratory failure).
With access to the oral cavity and ongoing pre-oxygenation, AIM replaces the often rushed and injury-prone maneuvers with a seamless conversion to general anesthesia, using a supraglottic device or endotracheal intubation.


Eliminates Bite-blocks
AIM eliminates the need for the endoscopy bite blocks and the pink airways for awake fiberoptic intubations, minimizing patient discomfort, reducing exposure to contaminated objects, and medical waste disposal.
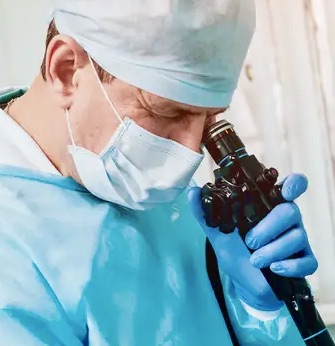
Awake Fiberoptic Intubation
AIM may facilitate awake fiberoptic intubation through its unique advantages. Elimination of the conventional pink-airway decreases patient discomfort (gag reflex), while continuous pharyngeal oxygen delivery reduces the risk of hypoxemia.
AIM may be reintroduced before extubation to provide high FiO₂ for a safer transition to spontaneous ventilation.
May Lower the Risk of Airway Fires
Nasal cannulas and face masks accumulate oxygen around patient’s face (oxygen is heavier than air), risking airway fires, patient injuries and malpractice lawsuits.
AIM’s oxygen delivery into the oro-pharynx may help reduce the risk of airway fires.


Small Size, Big Impact
Despite its small size, the high oxygen delivery, reliable capnography, single-step airway resuscitation and seamless conversion to general anesthesia make AIM an ideal device for a broad range of NORA procedures.
Lower Environmental Impact
Due to it’s compact size, AIM consumes fewer manufacturing resources.
Additionally, by eliminating the need for EGD and TEE bite-blocks and awake fiberoptic intubation (pink) airways, AIM further lowers disposable consumption.


Manufactured in the USA
With a focus on manufacturing the highest quality medical device, everything from the device molds, sourcing raw materials, manufacturing, and packaging is done here in the US.
No overseas containers, and no supply chain issues.
